UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d)
of The Securities Exchange Act of 1934
Date of Report (Date of Earliest Event Reported): January 14, 2021
ALLOVIR, INC.
(Exact name of registrant as specified in its charter)
| Delaware | 001-39409 | 83-1971007 | ||
| (State or other jurisdiction of incorporation) |
(Commission File Number) |
(I.R.S. Employer Identification No.) |
AlloVir, Inc.
139 Main Street, Suite 500
Cambridge, Massachusetts 02142
(Address of principal executive offices, including zip code)
(617) 433-2605
(Registrant’s telephone number, including area code)
Not Applicable
(Former Name or Former Address, if Changed Since Last Report)
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ☐ | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ☐ | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ☐ | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ☐ | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class |
Trading Symbol(s) |
Name of each exchange on which registered | ||
| Common stock, $0.0001 Par Value | ALVR | Nasdaq Global Select Market |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 or Rule 12b-2 of the Securities Exchange Act of 1934.
Emerging growth company ☒
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☐
| Item 7.01 | Regulation FD Disclosure. |
AlloVir, Inc. (the “Company”) is furnishing a corporate presentation, attached as Exhibit 99.1 to this Current Report on Form 8-K, which the Company intends to use from time to time in meetings with investors and others beginning on January 14, 2021. The corporate presentation will also be available in the investor relations section of the Company’s website at http://allovir.com.
The information in this Item 7.01 and Exhibit 99.1 attached hereto shall not be deemed “filed” for purposes of Section 18 of the Securities and Exchange Act of 1934, as amended, or otherwise subject to the liabilities of that section, nor shall they be deemed incorporated by reference in any filing under the Securities Act of 1933, as amended, except as expressly set forth by specific reference in such filing.
| Item 9.01 | Financial Statements and Exhibits. |
(d) Exhibits
| Exhibit |
Description | |
| 99.1 |
AlloVir, Inc. corporate presentation. | |
SIGNATURE
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| AlloVir, Inc. | ||||||
| Date: January 14, 2021 | By: | /s/ David Hallal | ||||
| David Hallal | ||||||
| Chief Executive Officer | ||||||

A Leader in Allogeneic, Off-the-Shelf Virus-Specific T-Cell Immunotherapies Exhibit 99.1

Disclaimer This presentation has been prepared by AlloVir, Inc. (“we,” “us,” “our,” “AlloVir” or the “Company”) and is made for informational purposes only and does not constitute an offer to sell or a solicitation of an offer to buy securities. This presentation may contain “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995, including, but not limited to, statements regarding the safety, efficacy and regulatory and clinical progress of our product candidates, including Viralym-M. All such forward-looking statements are based on management’s current expectations of future events and are subject to a number of risks and uncertainties that could cause actual results to differ materially and adversely from those set forth in or implied by such forward-looking statements. The use of words such as, but not limited to, “may,” “might,” “will,” “should,” “expect,” “plan,” “anticipate,” “believe,” “estimate,” “project,” “intend,” “future,” “potential,” or “continue,” and other similar words or expressions are intended to identify forward-looking statements. Forward-looking statements are neither historical facts nor assurances of future performance. Instead, they are based on our current beliefs, expectations and assumptions regarding the future of our business, future plans and strategies, our clinical results and other future conditions. All statements other than statements of historical facts contained in this presentation, including statements regarding future results of operations and financial position, business strategy, current and prospective product candidates ongoing, and planned clinical trials and preclinical activities, including the initiation, timing, enrollment, progress and results of our preclinical and clinical studies and our research and development programs, product approvals, research and development costs, current and prospective collaborations, timing and likelihood of success, including our ability to advance and successfully complete clinical studies, the timing and likelihood of success of our clinical trials, and the timing or likelihood of regulatory filings and approvals, expectations regarding market acceptance and size, plans for launch and commercialization, plans and objectives of management for future operations, and future results of anticipated product candidates, are forward-looking statements. New risks and uncertainties may emerge from time to time, and it is not possible to predict all risks and uncertainties. No representations or warranties (expressed or implied) are made about the accuracy of any such forward-looking statements. These statements are also subject to a number of material risks and uncertainties that are discussed in the section entitled "Risk Factors" in our quarterly report on Form 10-Q for the fiscal quarter ended September 30, 2020, as well as discussions of potential risks, uncertainties, and other important factors in our subsequent filings with the Securities and Exchange Commission. Any forward-looking statement speaks only as of the date on which it was made. Neither we, nor our affiliates, advisors or representatives, undertake any obligation to publicly update or revise any forward-looking statement, whether as a result of new information, future events or otherwise, except as required by law. Certain information contained in this presentation relates to or is based on studies, publications, surveys and other data obtained from third-party sources and the Company’s own internal estimates and research. While we believe these third-party sources to be reliable as of the date of this presentation, we have not independently verified, and we make no representation as to the adequacy, fairness, accuracy or completeness of any information obtained from third-party sources. In addition, all of the market data included in this presentation involves a number of assumptions and limitations, and there can be no guarantee as to the accuracy or reliability of such assumptions. Finally, while we believe our own internal research is reliable, such research has not been verified by any independent source.

AlloVir: A Leading Innovator in Allogeneic, Off-the-Shelf Virus-Specific T Cell Immunotherapies Versatile platform with >275 clinical patients dosed and positive data generated Robust development plan with 3 ongoing pivotal/POC trials and 5 additional in 2021 With 93% response Viralym-M received RMAT & PRIME from FDA and EMA Large Global Market Opportunity for Viralym-M alone Manufacturing expertise leading to efficiencies of scale Robust IP and patent portfolio covering both products and processes Experienced & proven developmental, clinical, manufacturing and commercial leadership team RMAT: Regenerative medicine advanced therapy; PRIME: PRIority MEdicine; POC: proof-of-concept Catalyst rich story with multiple data readouts expected each year

Leadership Team Led by an Experienced Management Team with a Strong Operating and Scientific Foundation Ann Leen, Ph.D. Chief Scientific Officer Co-Founder AlloVir Professor, BCM CAGT David Hallal Chief Executive Officer CEO of ElevateBio Former CEO Alexion Amgen Jeroen van Beek, Ph.D. Chief Commercial Officer Former CCO Tricida Alexion, Pfizer Agustin Melian, M.D. Chief Medical Officer Former SVP Alexion Merck Vikas Sinha, MBA President & Chief Financial Officer CFO of ElevateBio Former CFO Alexion Bayer Edward Miller, J.D. General Counsel Former SVP Alexion Boehringer Ingelheim Ercem Atillasoy, M.D. Chief Regulatory & Safety Officer Former VP Global Regulatory Affairs & Clinical Safety, Merck Dana Alexander SVP of CMC Operations Former Head of Viral Vectors Brammer Bio Medha Chadha SVP Strategic Planning and IR Former US Head of Equity Capital Markets Syndicate Cantor Fitzgerald

High Risk Populations with T Cell Deficiencies are Vulnerable to Life-Threatening Viral Diseases Despite Current Treatment Options PML: Progressive multifocal leukoencephalopathy; HCC: hepatocellular carcinoma 1. Abudayyeh A, et al. Am J Transplant. 2016;16:1492-1502. 2. Camargo JF, Komarduri KV. Hematol Oncol Stem Cell Ther. 2017;10:233-238. 3. Cesaro S, et al. Bone Marrow Transplant. 2018;doi:10.1038/s4109-018-0421-0. 4. Leen AM, et al. Blood. 2009;114(19):4283-4292. 5. Perruccio K, et al. Biol Blood Marrow Transplant. 2018;24:2649-2557. 6. Saribas AS, et al. Future Virol. 2010;5(3):313-323. doi:10.2217/fvl.10.12. 7. Cho SY, et al. Kor J Intern Med. 2018;33:256-276. 8. Law N, Kumar D. Drugs Aging. 2017;34:743-754. 9. Gentile G, Antonelli G. Viruses. 2019;11:doi:10.3390/v11111049. 10. Luppi M, et al. New Engl J Med. 2000;343:1378-1385. 11. Kedia S, et al. J Stem Cell Res Ther. 2013;doi:10.4172/2157-7633.S3-002. 12. Ison MG, Hirsch HH. Clin Microbiol Rev. 2019;32(4):1-33. 13. Jose RJ, et al. Medicine. doi:10.1016/j.mpmed.2020.03.006. 14. Simon AK, Hollander GA, McMichael A. Proc Biol Sci. 2015;282(1821):20143085. Viruses with No / Limited Treatments Brain Seizure Severe encephalitis Memory defect PML Eyes Retinitis Blindness Bladder Severe hemorrhagic cystitis Urinary obstruction Cystectomy Small/Large Intestine Colitis Ulceration / perforation Intestinal bleeding Liver Chronic hepatitis Liver cirrhosis HCC Malignancy Kaposi sarcoma Primary Effusion Lymphoma Kidneys Nephritis Acute/chronic renal failure End stage renal disease Lungs Pneumonia Bronchitis Respiratory failure High-risk populations BK virus1 Cytomegalovirus2 Adenovirus3 Epstein-Barr virus4 Human Herpesvirus-65 JC Virus6 Respiratory Syncytial Virus7 Parainfluenza Virus7 Human Metapneumovirus8 Influenza Virus7 SARS-CoV-28 Hepatitis B Virus9 Human Herpesvirus-810 Uncontrolled Viral Diseases Cause End-Organ Damage and Mortality1-3,10-13 HSCT Patients11,12 SOT Patients12 Elderly14 Very Young14 Other13 (e.g. Cancer)

AlloVir Has a Deep Pipeline of 5 Allogeneic, Off-the-Shelf VST Investigational Therapies Targeting 12 Viruses POC: Proof-of-concept; Allo-HSCT: Allogeneic HSCT; Auto-HSCT: Autologous HSCT; KT: Kidney Transplant; SOT: Solid Organ Transplant; KS: Kaposi Sarcoma; MCD: Multicentric Castleman Disease; PEL: Primary Effusion Lymphoma THERAPY CANDIDATE TARGET INDICATION TARGET POPULATION PRECLINICAL POC TRIAL (Phase 1b/2) PIVOTAL TRIAL (Phase 3) Viralym-M (ALVR105) Multi-VST Treatment of Virus-Associated Hemorrhagic Cystitis Allo-HSCT Treatment of CMV Treatment of AdV Prevention of BKV, CMV, AdV, EBV, HHV-6 and JCV Treatment of BKV Kidney Transplant Treatment of CMV Solid Organ Transplant ALVR106 Multi-VST Treatment of RSV, Influenza, PIV, and hMPV Allo- / Auto-HSCT High-risk General Population ALVR109 Single-VST Treatment of COVID-19 High-risk General Population ALVR107 Single-VST Treatment of HBV Patients with Chronic HBV ALVR108 Single-VST Treatment of HHV-8 Patients with KS, MCD or PEL IND Cleared Q4 2020 IND Cleared Q3 2020

Key Investment Highlights VERSATILE ENGINE for allogeneic, off-the-shelf, virus-specific T-cell immunotherapies TARGETING 12 devastating and life-threatening viruses WITH NO OR LIMITED TREATMENTS CLINICALLY VALIDATED LEAD PROGRAM, VIRALYM-M, targeting multiple indications 93% overall response rate demonstrated in Ph 2 trials Large market opportunity in RMAT / PRIME designated indications alone 3 pivotal and 3 POC trials initiated by end of 2021 ROBUST PIPELINE with an additional 4 VSTs in various stages of development ALVR109, for the treatment of COVID-19, expecting initial data in 2021 ALVR106, multi-respiratory VST, POC trial initiation expected in 2021 ALVR107/108, HBV/HHV8 VSTs, each targeting additional large addressable patient populations CATALYST RICH story with 2-3 data read outs expected every year EXPERIENCED MANAGEMENT TEAM

Transplant Patients and Viral Diseases

Nearly Two-Thirds of Allogeneic HSCT Recipients Have More Than One dsDNA Viral Infection CMV: Cytomegalovirus; AdV: Adenovirus; EBV: Epstein-Barr virus; HHV6: human herpesvirus 6. Hill et al, Blood 2017. Incidence of Viral Infections BKV, CMV, AdV, EBV and HHV-6 100% 90% 80% 70% 60% 50% 40% 30% 20% 10% 0 0 10 20 30 40 50 60 70 80 90 100 Cumulative Incidence Probability Days After HSCT A 37% Increase of Non-Relapse Mortality for Every Log Increase in Viral Load from Day 1-100 in Allogeneic HSCT Patients ≥ 1 virus ≥ 2 viruses ≥ 3 viruses ≥ 4 viruses

Virus-Associated Hemorrhagic Cystitis in HSCT: A Devastating Disease with No Approved or Effective Treatment Options HC, a common manifestation in HSCT, caused by BKV, AdV and/or CMV *Treatment related mortality 1. Cesaro S, et al. J Antimicrob Chemother. 2018;73:12–21. 2. Garguilo et al, ecancer. 2014; 8:420 doi: 10.3332/ecancer.2014.420. 3. Silva LdeP, et al. Haematologica. 2009;95(7):1183-1190. 4. Kloos RQ, et al. Biol Blood Marrow Transplant. 2013;19(8):1263-1266. 5. Type B Briefing Package. 6. Laskin BL, et al. Clin Infect Dis. 2019. doi: 10.1093/cid/ciz1194; 7. Gilis L, et al. Bone Marrow Transplantation. 2014;49: 664–670. Severe bleeding due to hematuria Severe, prolonged and intractable pain Increased mortality* HC Results in Severe Morbidity & Mortality1-7 No Approved or Effective Therapies1-7 RBC or platelet transfusions Bladder arteriole embolization and/or cystectomy Narcotics Life-disturbing urinary symptoms Continuous bladder irrigation Kidney dysfunction / failure Dialysis

Cytomegalovirus and Adenovirus in HSCT: Cause Severe and Life-Threatening Consequences 1. Hill J, et al. Blood 2017; 2. Ljungman P, et al. Clin Infect Dis. 2017; 3. Marty F, et al. NEJM 2017; 4. Sedláček P, et al. Biol Blood Marrow Transplant 2018; 5. Lion et al. Clin Microbiol Rev 2014 6. other than for CMV retinitis CMV Affects 65% of allogeneic HSCT patients1 Potentially life-threatening consequences2 Pneumonia Colitis Retinitis Encephalitis Multi-organ failure/Death No FDA- or EMA-approved anti-viral agents6 Off-label antiviral use associated with severe toxicities, including myelosuppression and nephrotoxicity Discontinuation of letermovir increased CMV infection (~18%) >100 days post HSCT3 AdV Occurs in 32% of pediatric and 6% of adult allogeneic HSCT patients4 Potentially life-threatening consequences5 Pneumonia Hemorrhagic enteritis or cystitis Hepatitis Multi-organ failure/Death No FDA-or EMA approved treatments Off-label antiviral use agent has demonstrated limited efficacy and severe toxicities including nephrotoxicity

Worse Graft Survival in SOT Patients with Persistent CMV Infection2 BKV in Kidney Transplant & CMV in SOT Patients: Lead to Decreased Graft Survival Despite Standard of Care1,2 BKVN: BKV Nephropathy 1. Vasudev B, et al. Kidney International 2005; 2. Legendre C and Pascual C. CID 2008 Worse Graft Survival in KT Patients with BKV Nephropathy1 40% 50% 60% 70% 80% 90% 100% 0 0.5 1 2 3 4 5 Patients with BKVN Patients without BKVN P < 0.001 Post-transplant years Graft Survival, % P = .020 5 4 3 2 1 0 Non-persistent or no CMV Persistent CMV Post-transplant years Graft Survival, % 40% 50% 60% 70% 80% 90% 100%

Our Solution Allogeneic, Off-the-Shelf Virus-Specific T-Cells

Treated with Current Care Options Our Approach Utilizes the Adoptive Transfer of Off-the-Shelf VSTs to Restore Virus-specific Immunity1-6 1. Swain, S., et al. Nat Rev Immunol 2012.; 2. Muraro E, et al. Front. Immunol. 2017; 3. Rosendahl HS et al. Front Immunol. 2014. 4. Tzannou, JCO 2017; 5. Vasileiou S et al. Haematol 2019. 6. Type B Meeting Briefing Package. Patients with T-cell Deficiency are at High Risk for Devastating Viral Infections Deficient T-cell Immunity Fails to Control Viral Infections, Resulting in Devastating Consequences Treated with Off-the-Shelf VSTs Restored T-cell Immunity with Expanded Off-the-Shelf VSTs Controls Viral Infections Virus-infected cells Expanded Off-the-Shelf VSTs CD8+ Off-the-Shelf VSTs CD4+ Off-the-Shelf VSTs CD4+ T cells in patients CD8+ T cells in patients

Our Patented, Highly Efficient and Industrialized Platform Provides Key Advantages1-5 *Patent pending | PBMCs: Peripheral Blood Mononuclear Cells 1. Tzannou I, et al. Blood Adv. 2019;3(17):2571–2580. 2. Tzannou I, et al. J Clin Oncol. 2017 Nov 1;35(31):3547-3557. 3. Vasileiou S, et al. Haematologica. 2019 Apr. 4. Saglio et al. Cytotherapy. 2014 February; 16(2): 149–159. 5. Type B Briefing Package. Mini-Banks of Cryopreserved VSTs Step 1 VST Profiling / Antigen Selection & Donor Selection (CytokinTM*) Step 3 CytomatchTM* and Immediate Access to Our Allogeneic VST Therapies Step 2 Rapid and Scalable Off-the-Shelf VST Manufacturing (14 ± 2 Days in Viralym-M) Selective Expansion of VSTs Using Proprietary Process Carefully Selected Healthy Seropositive Donors PBMCs from Donors VST Profiling & Antigen Selection + Selected Antigens CytomatchTM* to rapidly select best-fit VSTs for patients VSTs have long-term stability and are available on demand as off-the-shelf therapy Proprietary VST profiling and antigen selection* process Immediate availability of off-the-shelf VSTs CytokinTM* ensures broad patient coverage with a targeted number of carefully screened donor pool One manufacturing run from a single donor can generate hundreds of doses of VSTs Patented expansion process designed to prevent antigen competition and preserve polyclonality of VSTs Mini-banks ensure >95% patient coverage Patient with Viral Infection CytomatchTM* Treatment of Patient CytokinTM*

Viralym-M (ALVR105) The Potential to Transform the Lives of Transplant Patients by Dramatically Improving or Preventing Morbidity and Mortality

Stimulation and Expansion of Polyclonal VSTs in One-step, High-yield Process PBMCs from Donors Identified by Proprietary Process Viralym-M: Our VST Therapy Designed to Target Viral Diseases That Result in Significant Morbidity and Mortality Post Allogeneic HSCT PBMCs: Peripheral Blood Mononuclear Cells 12 Selected Antigens for Viralym-M + PBMCs Stimulation & Expansion Antigens Selected by Proprietary Virus Profiling and Antigen Selection Process Viralym-M (ALVR105) Multi-VST targeting BKV, CMV, AdV, EBV, and HHV-6 BKV: LT, VP1 CMV: IE1, pp65 AdV: Hexon, Penton EBV: EBNA1, LMP2, BZLF1 HHV-6: U11, U14, U90
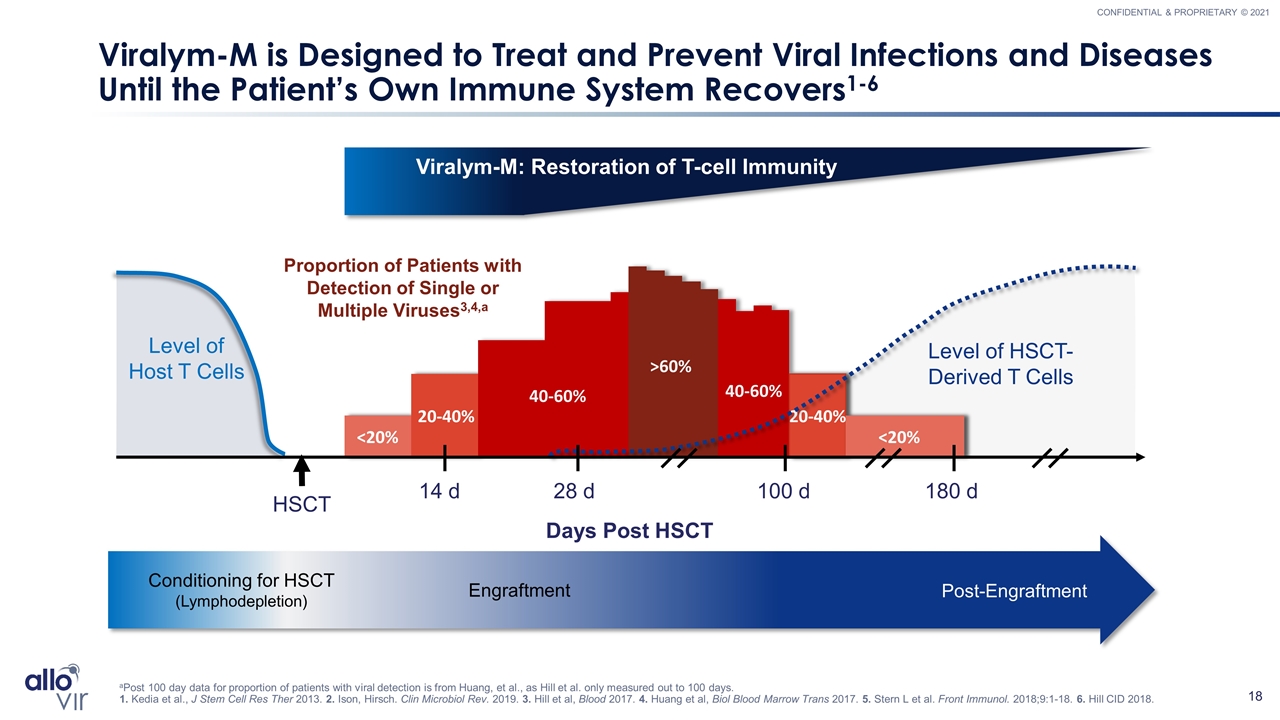
<20% 20-40% 40-60% >60% 40-60% <20% 20-40% Viralym-M is Designed to Treat and Prevent Viral Infections and Diseases Until the Patient’s Own Immune System Recovers1-6 aPost 100 day data for proportion of patients with viral detection is from Huang, et al., as Hill et al. only measured out to 100 days. 1. Kedia et al., J Stem Cell Res Ther 2013. 2. Ison, Hirsch. Clin Microbiol Rev. 2019. 3. Hill et al, Blood 2017. 4. Huang et al, Biol Blood Marrow Trans 2017. 5. Stern L et al. Front Immunol. 2018;9:1-18. 6. Hill CID 2018. Level of Host T Cells Conditioning for HSCT (Lymphodepletion) Engraftment Post-Engraftment Level of HSCT-Derived T Cells Days Post HSCT HSCT 14 d 100 d 180 d 28 d Viralym-M: Restoration of T-cell Immunity Proportion of Patients with Detection of Single or Multiple Viruses3,4,a

Phase 2 POC CHARMS Trial Design (N=59*) Viralym-M Phase 2 Proof-of-Concept Study, CHARMS, Generated Promising Preliminary Disease Outcome and Safety Data Phase 2, proof-of-concept, open label study to assess the safety and clinical effects of Viralym-M in allogeneic HSCT recipients with ≥1 treatment-refractory Infections The CHARMS trial treated 58 unique patients. One patient was counted twice: enrolled twice, treated first for AdV and then for JCV. One patient with HHV-6 was not evaluable for response rate GVHD: graft vs host disease. 1. Tzannou, JCO 2017; 2. Tzannou I, et al. Treatment of severe, drug-refractory viral infections with allogeneic, off-the-shelf multi-virus specific T cells in patients following HSCT: results from a phase 2 study. Paper presented at: 62nd ASH Annual Meeting and Exposition. December 5–8, 2020. Accessed January 4, 2021. https://ash.confex.com/ash/2020/webprogram/Paper143037.html Population Refractory BKV, CMV, AdV, EBV, HHV-6 and/or JCV Failure of antiviral therapy; OR Unable to tolerate standard antivirals Day1 Single infusion 6 weeks Assessment of clinical endpoints Study endpoints Safety Clinical endpoints Viral loads Clinical & virologic responses Follow-up 12 months End of study Viralym-M (ALVR105):

Viralym-M was Generally Well Tolerated in CHARMS Trial (N=59*)1-2 *The CHARMS study treated 59 patients (one patient was counted twice: enrolled twice, treated first for AdV and then for JCV) GI: gastrointestinal; GVHD: graft versus host disease 1. Tzannou, JCO 2017; 2. Tzannou I, et al. Treatment of severe, drug-refractory viral infections with allogeneic, off-the-shelf multi-virus specific T cells in patients following HSCT: results from a phase 2 study. Paper presented at: 62nd ASH Annual Meeting and Exposition. December 5–8, 2020. Accessed January 4, 2021. https://ash.confex.com/ash/2020/webprogram/Paper143037.html Infusions were well tolerated Three patients developed an isolated fever within 24 hours of infusion, no immediate toxicities were observed There were 14 cases of acute GVHD 8 patients with pre-existing GVHD 6 patients with de novo GVHD; All had transient Grade I skin GVHD resolved with treatment No patients developed cytokine release syndrome
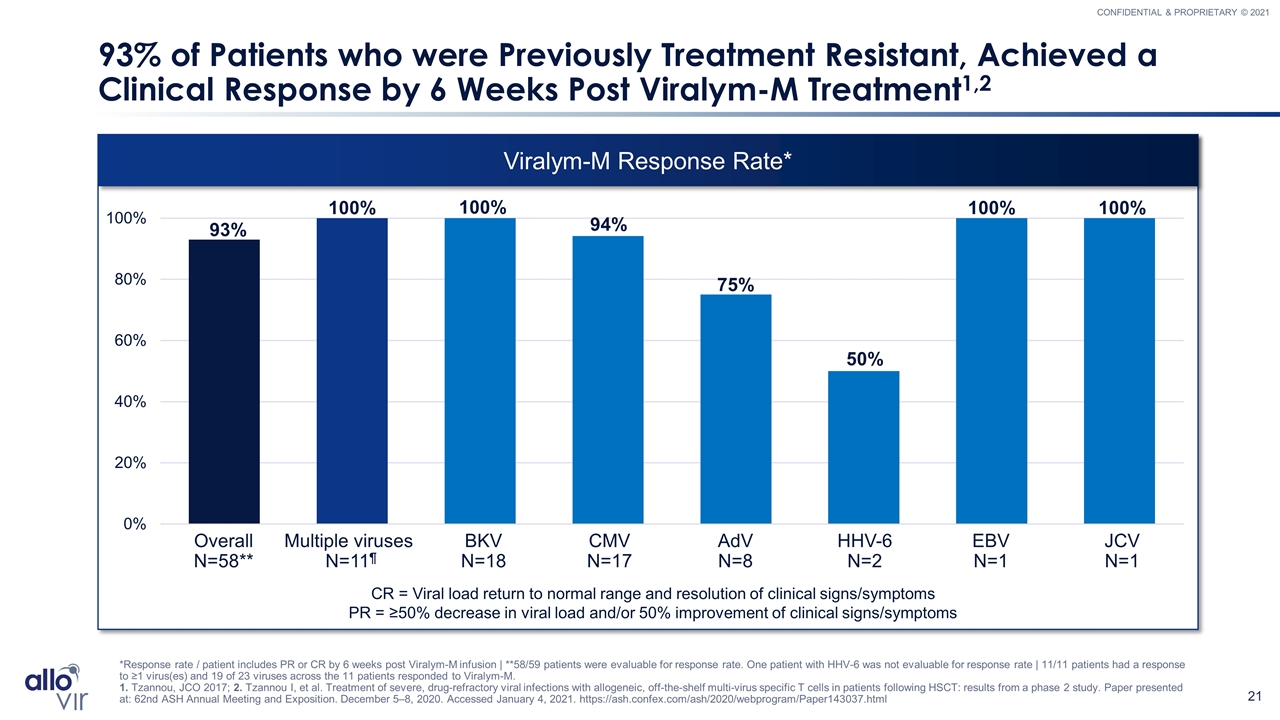
Viralym-M Response Rate* 93% of Patients who were Previously Treatment Resistant, Achieved a Clinical Response by 6 Weeks Post Viralym-M Treatment1,2 *Response rate / patient includes PR or CR by 6 weeks post Viralym-M infusion | **58/59 patients were evaluable for response rate. One patient with HHV-6 was not evaluable for response rate | 11/11 patients had a response to ≥1 virus(es) and 19 of 23 viruses across the 11 patients responded to Viralym-M. 1. Tzannou, JCO 2017; 2. Tzannou I, et al. Treatment of severe, drug-refractory viral infections with allogeneic, off-the-shelf multi-virus specific T cells in patients following HSCT: results from a phase 2 study. Paper presented at: 62nd ASH Annual Meeting and Exposition. December 5–8, 2020. Accessed January 4, 2021. https://ash.confex.com/ash/2020/webprogram/Paper143037.html Multiple viruses N=11¶ HHV-6 N=2 AdV N=8 EBV N=1 CMV N=17 Overall N=58** BKV N=18 CR = Viral load return to normal range and resolution of clinical signs/symptoms PR = ≥50% decrease in viral load and/or 50% improvement of clinical signs/symptoms JCV N=1

Virus-Associated Hemorrhagic Cystitis: Rapid Resolution was Achieved in Patients Treated with Viralym-M BKV-HC: BKV-associated hemorrhagic cystitis *Resolution of BKV-HC: Grade 1 (microscopic hematuria; minimal increase in frequency, urgency, dysuria, or nocturia; new onset of incontinence) or 0 (no symptoms) **Based on 20 patients, including pediatric and adult patients, with data available for HC grading retrospectively assessed by 3 independent physicians using NCI CTCAE v4 Source: Type B Briefing Package Percent Resolved* Patients with BKV-HC Treated with Viralym-M in CHARMS Trial (N=20)**

Virus-Associated Hemorrhagic Cystitis: Prolonged Symptomatic Disease Observed in Patients Treated with SOC BKV-HC: BKV-associated hemorrhagic cystitis; SOC: standard of care *Resolution of BKV-HC: Grade 1 (microscopic hematuria; minimal increase in frequency, urgency, dysuria, or nocturia; new onset of incontinence) or 0 (no symptoms) **In a retrospective study conducted at Baylor College of Medicine, out of the 33 pediatric allogeneic HSCT patients with an average of Grade 3 BK-HC receiving current standard of care, unpublished Source: Type B Briefing Package Natural History Cohort Treated with Standard of Care (N=33)** Percent Resolved*
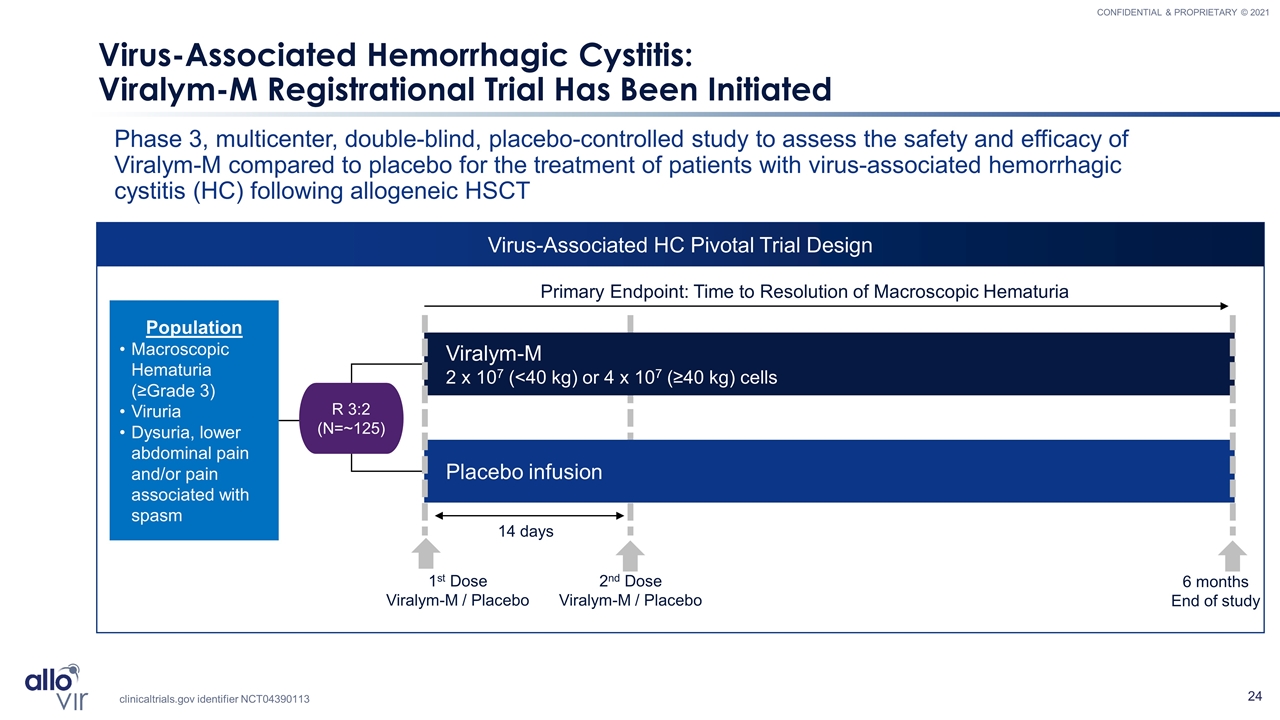
Virus-Associated HC Pivotal Trial Design Virus-Associated Hemorrhagic Cystitis: Viralym-M Registrational Trial Has Been Initiated Phase 3, multicenter, double-blind, placebo-controlled study to assess the safety and efficacy of Viralym-M compared to placebo for the treatment of patients with virus-associated hemorrhagic cystitis (HC) following allogeneic HSCT Population Macroscopic Hematuria (≥Grade 3) Viruria Dysuria, lower abdominal pain and/or pain associated with spasm Viralym-M 2 x 107 (<40 kg) or 4 x 107 (≥40 kg) cells Placebo infusion R 3:2 (N=~125) Primary Endpoint: Time to Resolution of Macroscopic Hematuria 2nd Dose Viralym-M / Placebo 6 months End of study 1st Dose Viralym-M / Placebo 14 days Clinical Trials.gov NCT04401410 clinicaltrials.gov identifier NCT04390113

11/11 (100%) patients in CHARMS trial had a response to ≥1 virus(es) 19 of 23 viruses across the 11 patients responded to Viralym-M Multiple-viruses: Viralym-M Achieved 100% Response in Patients with ≥2 Viruses 1. Tzannou, JCO 2017; 2. Tzannou I, et al. Treatment of severe, drug-refractory viral infections with allogeneic, off-the-shelf multi-virus specific T cells in patients following HSCT: results from a phase 2 study. Paper presented at: 62nd ASH Annual Meeting and Exposition. December 5–8, 2020. Accessed January 4, 2021. https://ash.confex.com/ash/2020/webprogram/Paper143037.html 11 Patients with 23 Viral Infections

Viralym-M 2 x 107 (<40 kg) or 4 x 107 (≥40 kg) cells Multi-Virus Prevention POC Study Design Multi-Virus Prevention: Viralym-M POC Has Been Initiated with Initial Data Expected in 2021 Phase 2, multicenter, randomized, double-blind, placebo-controlled study to assess the safety and efficacy of Viralym-M compared to placebo for the prevention of AdV, BKV, CMV, EBV, HHV-6, and JCV infection and/or disease, in high-risk patients after allogeneic hematopoietic cell transplant Population High risk patients post allogeneic HSCT Cohort A (preemptive): Positive for at least one viruses Cohort B (prophylaxis): undetectable for all six viruses 6 months End of study Placebo infusion R 1:1 (N=~125) Viralym-M Open Label (N=25) 2 x 107 (<40 kg) or 4 x 107 (≥40 kg) cells Primary Endpoint: Reduction in clinically-significant viral infection and disease Week 14 efficacy analysis Week 14 efficacy analysis 6 month safety follow-up Week 4 operational review Initiation of Placebo-Controlled Phase clinicaltrials.gov identifier NCT04693637
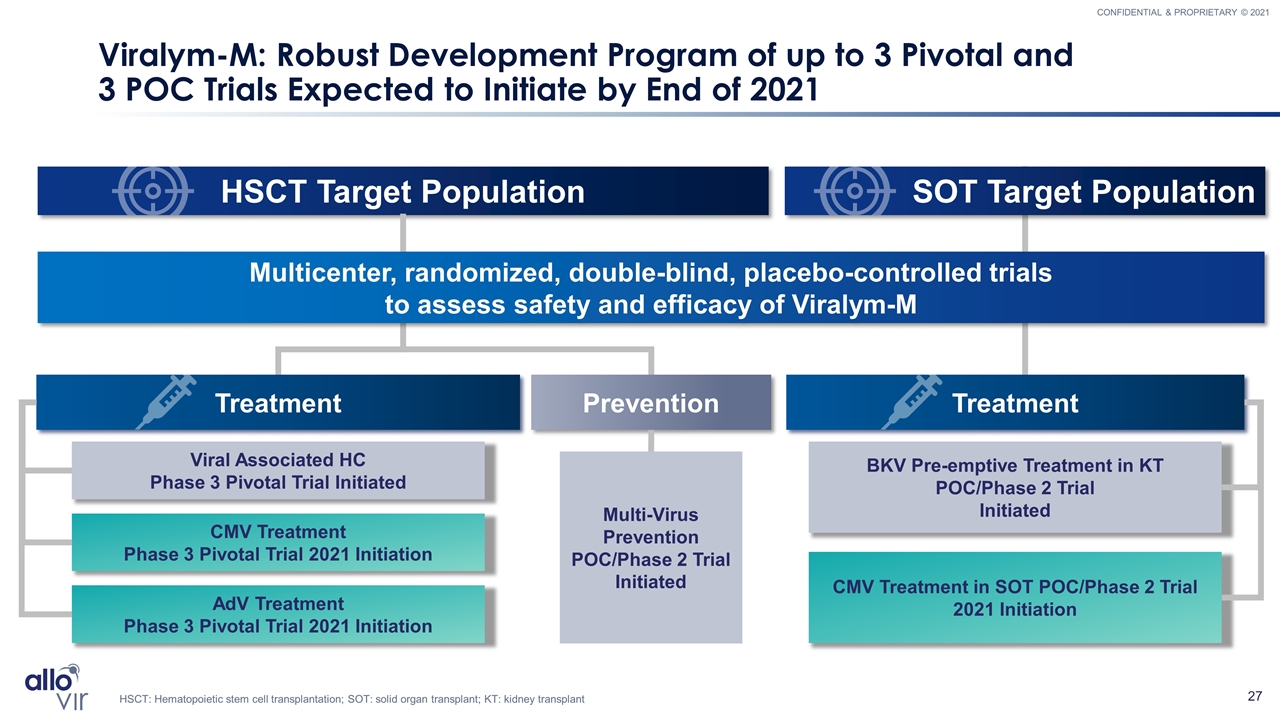
Viralym-M: Robust Development Program of up to 3 Pivotal and 3 POC Trials Expected to Initiate by End of 2021 CMV Treatment Phase 3 Pivotal Trial 2021 Initiation Viral Associated HC Phase 3 Pivotal Trial Initiated Multi-Virus Prevention POC/Phase 2 Trial Initiated BKV Pre-emptive Treatment in KT POC/Phase 2 Trial Initiated CMV Treatment in SOT POC/Phase 2 Trial 2021 Initiation HSCT Target Population SOT Target Population Treatment Prevention Treatment Multicenter, randomized, double-blind, placebo-controlled trials to assess safety and efficacy of Viralym-M HSCT: Hematopoietic stem cell transplantation; SOT: solid organ transplant; KT: kidney transplant AdV Treatment Phase 3 Pivotal Trial 2021 Initiation

Viralym-M: Large Addressable Patient Population for the Treatment and Prevention of Devastating Viral Diseases Estimated Annual Addressable Patients in 2025 ~19,500 Treatment in HSCT ~40,000 Treatment and Prevention in HSCT ~97,000 Treatment in SOT Additional Patients Expansion beyond HSCT patients to SOT patients Prevention of multi-virus infections with Viralym-M will transform HSCT landscape Projected addressable patient population in 2025 for Viralym-M indications in target markets in NA, EU, LATAM and A/P *Projected addressable patient population in 2025 for Viralym-M indications in target markets in NA, EU, LATAM and A/P | Source: AlloVir analysis and estimates based on annual growth rate assumption Focused commercial infrastructure targeting high-volume transplant centers globally In US and EU5, 80% of allogeneic HSCT performed in top 70 / 185 and 129 / 411 stem cell transplant centers, respectively Top 100 / 240 transplant centers in US perform 80% of kidney transplants We believe that many of these transplant centers will also have participated in our pivotal and POC trials

HSCT Recipients with Virus-Associated HC Have Significantly Higher Mortality and Incur Greater Healthcare Reimbursements Real-world claims analysis confirms high clinical and economic burden of virus-associated hemorrhagic cystitis (HC) All reimbursed amounts in the insurance claims up to 1-year post-transplant were reported. Reimbursement amounts include inpatient services and admission summaries, outpatient services, and outpatient pharmaceutical dispensed claims | Source: Real-world claims data analysis 1-yr All-Cause Mortality Rate for Allogeneic HSCT Recipients Mean Total Reimbursements for Allogeneic HSCT Recipients

Extending Our Platform to Tackle Major Public Health Needs

Consequences to High-Risk Patients with URTI and LRTI Devastating Consequences of Respiratory Virus Infections and Disease Ison M and Hirsch H. CMR 2019. ALVR106 target viruses Progression from URTI to LRTI Lost productivity Fatigue ER visits Pneumonia Hospitalization Respiratory failure Death Potential Consequences Upper Respiratory Tract Infection (URTI) Lower Respiratory Tract Infection (LRTI) Human metapneumo virus Influenza virus Respiratory Syncytial virus Parainfluenza virus Coronavirus

Respiratory Virus Infections & Diseases in High-Risk Populations: Substantial Unmet Need for Treatment and Prevention RTI: Respiratory tract infection; LRTI: Lower tract respiratory infection 1. WHO; 2. Paes BA, et al. Can Respir J. 2011;18(2):e10‐e19; 3. Branche AR, et al. Semin Respir Crit Care Med. 2016;37(4):538‐554. 4. Shafagati N, Williams J. F1000Res. 2018;7:135. Published 2018 Feb 1 5. Clayville LR. P T. 2011;36(10):659‐684; 6. Ison M and Hirsch H. CMR 2019. 7. Versluys AB, Boelens JJ. Front Microbiol. 2018;9:2795. 8. Piñana J, Montoro J, Aznar C, et al. J Infect. 2020;80(3):333‐341. doi:10.1016/j.jinf.2019.12.022. Devastating Consequences of Respiratory Infections/ Disease No or Limited Care Options Available6 SARS-CoV-2: Vaccines & EUA therapies efficacy currently undetermined for transplant and/or high-risk populations PIV and hMPV: No FDA-/EMA-approved treatment or vaccines RSV: Ribavirin / pavilizumab for children / no vaccines available Logistical challenge to administer, toxicity, and development of resistance Influenza: neuraminidase inhibitors & vaccines Drug resistance common in immunocompromised patients Partially effective vaccine in high-risk populations High-risk populations SARS-CoV2: >76,000,000 confirmed cases of COVID-19 & >1,500,000 deaths worldwide as of December 22, 20201 RSV: ~ 66,000 – 199,000 deaths each year2 PIV: 7% of pediatric and up to 11.5% of adult hospitalization for RTIs3 hMPV: 50% of infected elderly patients developed LRTI, which led to 50% mortality4 Influenza: High mortality rates in patients ≥ 75 yrs and < 5 yrs5 Transplant population6-8 RTIs due to RSV, influenza, PIV and hMPV, detected in up to 40% of allogeneic HSCT patients ~50% progress to LRTI with 20-45% mortality rate Respiratory viruses can infect all types of SOT patients

ALVR106 & ALVR109 VST Therapies for Respiratory Viruses such as RSV, Influenza, PIV, hMPV and SARS-CoV-2

% Specific Lysis Effector : Target Ratio % Specific Lysis 40:1 Effector : Target Ratio Allogeneic targets Autologous targets N=8 N=8 No / Minimal Activity of ALVR106 against Non-Infected Targets 0% 10% 20% 30% 40% 50% 0% 20% 30% 40% 50% 10% Cytolytic Activity of ALVR106 against Target Viruses ALVR106, Multi-Respiratory Virus T-Cell Therapy Candidate Specific for RSV, Influenza, PIV, and hMPV, in High-Risk Patients with T Cell Deficiencies Vasileiou S et al. Haematologica 2020. ALVR106 has selective antiviral activity against target viruses while leaving non-virus infected targets intact
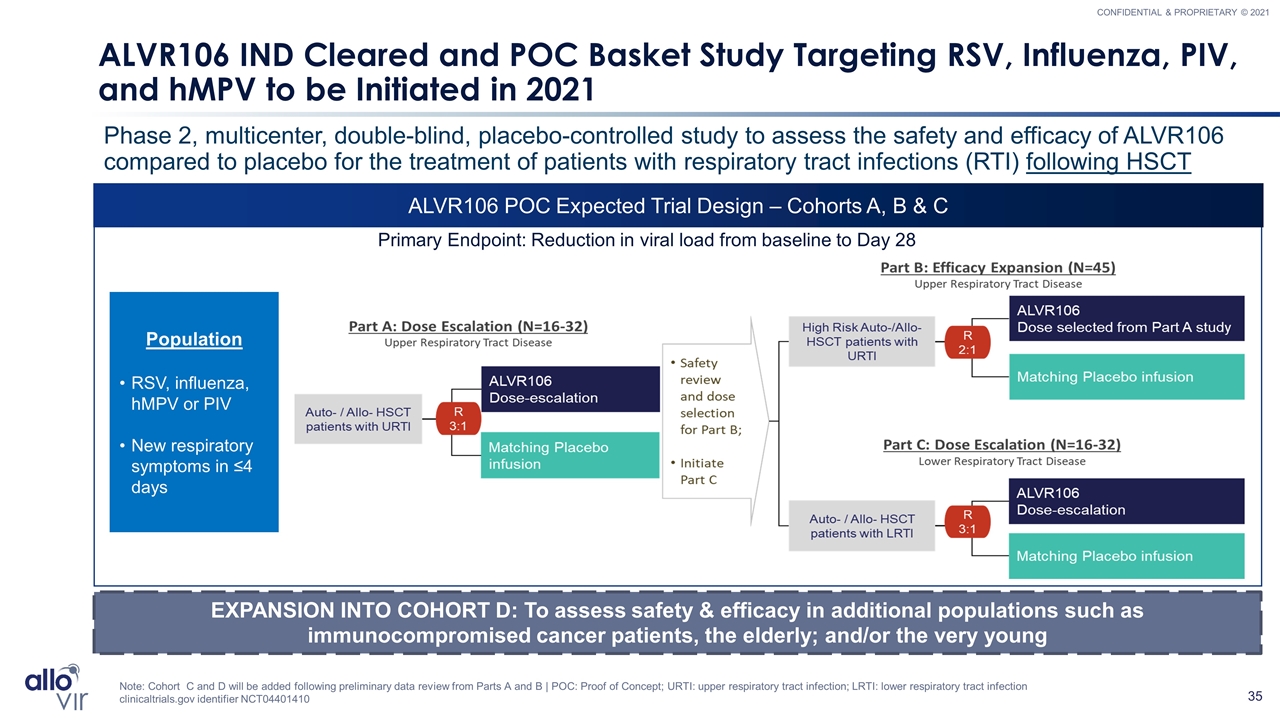
ALVR106 POC Expected Trial Design – Cohorts A, B & C ALVR106 IND Cleared and POC Basket Study Targeting RSV, Influenza, PIV, and hMPV to be Initiated in 2021 Phase 2, multicenter, double-blind, placebo-controlled study to assess the safety and efficacy of ALVR106 compared to placebo for the treatment of patients with respiratory tract infections (RTI) following HSCT EXPANSION INTO COHORT D: To assess safety & efficacy in additional populations such as immunocompromised cancer patients, the elderly; and/or the very young Note: Cohort C and D will be added following preliminary data review from Parts A and B | POC: Proof of Concept; URTI: upper respiratory tract infection; LRTI: lower respiratory tract infection clinicaltrials.gov identifier NCT04401410 Primary Endpoint: Reduction in viral load from baseline to Day 28 Population RSV, influenza, hMPV or PIV New respiratory symptoms in ≤4 days

High-Risk COVID-19 Patients Have Significant T-Cell Deficiencies **p<0.01, ***p<0.001, ****p<0.0001 Diao B, et al. Front Immunol 2020. T-cell Deficiencies in Different COVID-19 Patient Groups (N=522) T-cell Deficiencies in COVID-19 Patients of Different Ages (N=522) 1000 0 2000 3000 <20 y 20-59 yr ≥60yr Total T Cells **** **** **** Cells number/µl 1000 0 2000 3000 Healthy Non-ICU ICU Total T Cells **** **** ** Cells number/µl

ALVR109 Has Demonstrated Selective Cytolytic Activity against SARS-CoV-2 While Leaving Non-Virus Infected Targets Intact Source: ASH abstract: “Using Allogeneic, Off-the-Shelf, SARS-CoV-2-specific T Cells to Treat High Risk Patients with COVID-19” Cytolytic Activity of ALVR109 against SARS-CoV-2 No / Minimal Activity of ALVR109 against Non-Infected Targets % Specific Lysis Effector : Target Ratio Allogeneic targets Autologous targets N=4 % Specific Lysis -5% 0% 5% 10% 15% 20% 40:1 20:1 10:1 5:1 Effector :Target Ratio SARS-CoV-2 Control -5% 5% 35% 25% 45% 15%

ALVR109 POC Trial Initiated in Q4 2020 with Top Line Data Expected in 2021 MTD: Maximum tolerated dose *Including analyses of hospitalization, O2 requirements, need for mechanical ventilation and survival **Including Expansion/persistence and in vivo effects of infused T cells assessed by a range of T cell measures, endogenous immune reconstitution/antibody induction, extended safety of T cell infusion to day 28 and 42 post-infusion, etc. Clinical Trials.gov NCT04390113 ALVR109 POC Study Design Population COVID-19 patients with high risk of progression to mechanical ventilation Day1: Single infusion Safety follow-up 24 weeks: End of study ALVR109 1X107 cells X107 cells 4X107 cells Identification of MTD R 1:1 (N=40) 2 weeks: Endpoint assessment ALVR109 Optimized dose Standard of care Day1: Single infusion 2 weeks: Safety assessment Study endpoints Safety Clinical endpoints WHO Ordinal Scale* Exploratory endpoints**

Advancing Towards Commercialization

BaseCamp is a Premium Global Cell and Gene Therapy R&D and Manufacturing Company Dedicated to Its Affiliated Companies R&D for immunotherapy, regenerative medicine and gene therapy Process development GMP manufacturing of viral vectors GMP manufacturing of immune cells Regulatory and quality support Innovation and process consulting

AlloVir Has Achieved Meaningful Milestones in Off-the-Shelf VST Manufacturing Leveraging BaseCamp An external cGMP CDMO is currently manufacturing Viralym-M and ALVR106 An academic cGMP facility is manufacturing ALVR109 On track to add ElevateBio BaseCamp to our manufacturing network in 2021 Completed technology transfer of manufacturing process to our CDMO Successful engineering runs and potency assay to support multiple clinical trials Robust manufacturing process industrialized with CDMO GMP facility Quality control and computer system validation per FDA requirement have been completed

Conclusion
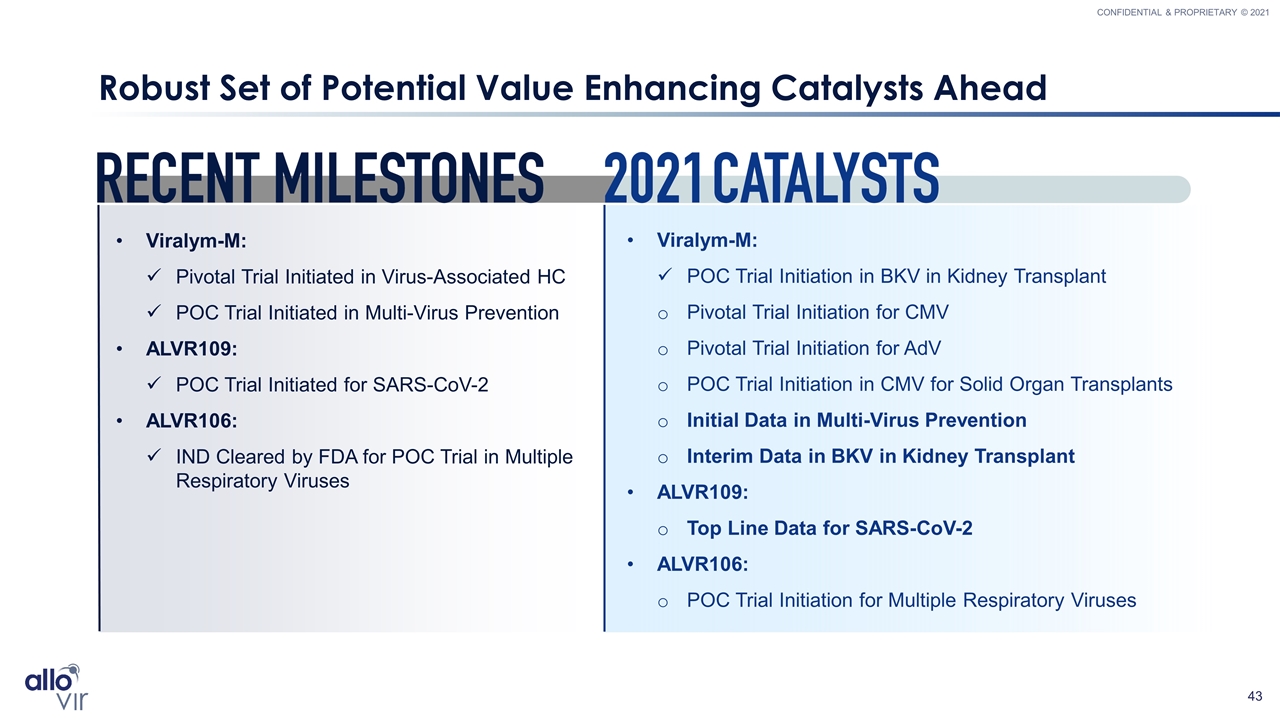
Robust Set of Potential Value Enhancing Catalysts Ahead Viralym-M: POC Trial Initiation in BKV in Kidney Transplant Pivotal Trial Initiation for CMV Pivotal Trial Initiation for AdV POC Trial Initiation in CMV for Solid Organ Transplants Initial Data in Multi-Virus Prevention Interim Data in BKV in Kidney Transplant ALVR109: Top Line Data for SARS-CoV-2 ALVR106: POC Trial Initiation for Multiple Respiratory Viruses Viralym-M: Pivotal Trial Initiated in Virus-Associated HC POC Trial Initiated in Multi-Virus Prevention ALVR109: POC Trial Initiated for SARS-CoV-2 ALVR106: IND Cleared by FDA for POC Trial in Multiple Respiratory Viruses

Key Investment Highlights VERSATILE ENGINE for allogeneic, off-the-shelf, virus-specific T-cell immunotherapies TARGETING 12 devastating and life-threatening viruses WITH NO OR LIMITED TREATMENTS CLINICALLY VALIDATED LEAD PROGRAM, VIRALYM-M, targeting multiple indications 93% overall response rate demonstrated in Ph 2 trials Large market opportunity in RMAT / PRIME designated indications alone 3 pivotal and 3 POC trials initiated by end of 2021 ROBUST PIPELINE with an additional 4 VSTs in various stages of development ALVR109, for the treatment of COVID-19, expecting initial data in 2021 ALVR106, multi-respiratory VST, POC trial initiation expected in 2021 ALVR107/108, HBV/HHV8 VSTs, each targeting additional large addressable patient populations CATALYST RICH story with 2-3 data read outs expected every year EXPERIENCED MANAGEMENT TEAM
